Proposed Traceability Rule
As part of the Food Safety Modernization Act (FSMA), the U.S. Food and Drug Administration (FDA) was tasked with enhancing the tracking and tracing and recordkeeping for high-risk foods. Section 204 of FSMA specifically requires that the FDA conduct traceability pilot projects, create a list of high-risk foods, and publish a proposed rule establishing extra recordkeeping requirements for high-risk foods. The FDA’s goal is to reduce the time it takes to respond to foodborne illness outbreaks thereby reducing the number of people who get sick. This article will examine how the FDA has addressed each of these requirements and further steps the agency has taken, particularly the recently released proposed rule.
Pilot Projects
While the FDA conducted the initial pilot projects mandated by FSMA in the timeframe allowed, FDA has continued with traceability pilots beyond those required by FSMA. The original pilot projects evaluated tomatoes and processed products which included chicken, peanuts and spices. These pilot programs, conducted by the Institute of Food Technologists (IFT), focused on how new traceability regulations would affect businesses, particularly small businesses. IFT made recommendations regarding the proposed rule to the FDA based on the findings of the pilot programs.
More recently, the FDA again enlisted IFT to conduct a traceability pilot regarding leafy greens as part of the New Era of Smarter Food Safety and the STEC Leafy Greens Action Plan. (See NALC’s blog post on Leafy Green Food Safety for more information). For this program, IFT focused on several products that contain romaine lettuce in different supply chains. For example, one of the pilots originated at a regional chain grocery store, one at an independent retailer that used a third-party distributor, and one at a national chain store. The pilots were conducted from July through October. Each pilot demonstrated that outbreak investigations can be simplified and more effective when extended traceback data is provided throughout the supply chain. In comparison, when conducting a recall currently, each member of the supply chain is only required to maintain “one up, one back” data. This means that each supply chain member only knows who sent them a product and who they then sent the product to. The extended traceback data expands this, requiring each supply chain member to maintain records of every step in the supply chain. This extended traceback data maintenance was accomplished through the use of a standard template called the “Produce Traceability Template” The template was used to exchange important information between suppliers. Feedback from participants in the study stated that while the template was helpful in knowing what information was being requested, the template needed to be better explained and simplified for future use.
These pilot programs will give FDA visibility into the supply chain so that the proposed rule can accommodate differences between suppliers and supply chains in an effort to ensure enhanced traceability and prompt recalls.
Food Traceability List
Another requirement under section 204 of FSMA is the designation of foods for which additional recordkeeping requirements are appropriate and necessary to protect public health. FSMA refers to these as “high-risk foods.” FDA solicited comments on a draft approach for developing a list of high-risk foods. Based on these comments and other information submitted, the agency created a risk-ranking model to identify high-risk foods that warrant inclusion on the Food Traceability List (FTL). The final Food Traceability List was published with the proposed rule and includes: cheeses, shell eggs, nut butter, cucumbers, fresh herbs, leafy greens, melons, peppers, sprouts, tomatoes, tropical tree fruits, fresh-cut fruits and vegetables, finfish, mollusk, and ready-to-eat deli salads. The proposed rule also includes a process to update the FTL through notice and comment rulemaking.
Proposed Rule
The proposed traceability rule, “Requirements for Additional Traceability Records for Certain Foods”, establishes the recordkeeping requirements for high-risk foods found on the FTL. The FDA intends that this rule will “standardize the data elements and information firms must establish and maintain, and the information they would need to send to the next entity in the supply chain to facilitate rapid and accurate traceability needed to prevent or mitigate foodborne illness outbreaks.”
The proposed rule requires that original records be maintained as either paper, electronic or true copies. Traceability records are required to be given to FDA upon request as soon as possible but not to exceed twenty-four hours from the request. Lastly, the rule requires, in most situations, that entities provide FDA an electronic sortable spreadsheet with relevant traceability information in the event of an outbreak, recall or other threat to public health.
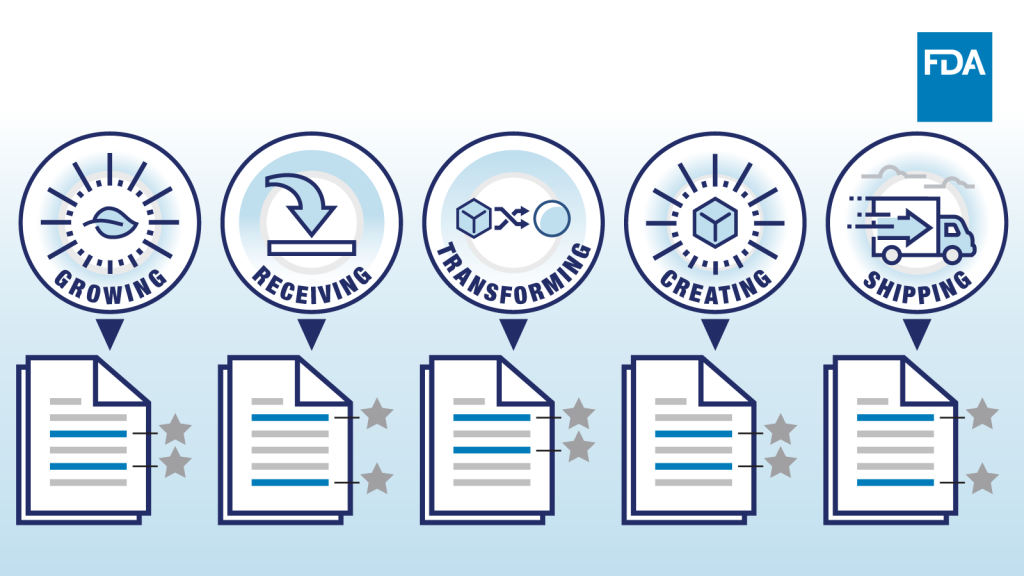
Key Data Elements
The proposed rule would require tracking Key Data Elements (KDEs) at each of the five Critical Tracking Events (CTEs): growing, receiving, transforming, creating, and shipping. At each CTE the responsible entity would need to record the traceability lot code, a uniquely identifiable code established when the food is originated, transformed or created, and relevant KDE. For growing, the required KDE is the growing area coordinates. However, there are some additional KDEs for sprouts. For receiving, the required KDEs relate to the location and quantity of the food received. However, if the person is the first receiver, that is, the first person who purchases and takes physical possession of a high-risk food, they are required to maintain extra information regarding the origination and initial handling of the food. There are also different requirements for first receivers of seafood. For transformation, where a food on the FTL is changed, repackaged or relabeled through combining ingredients, processing or packaging, the person is required to maintains records linking a new traceability lot code to KDEs involving location, quantity and records of transformation. For creation, where foods not on the FTL are combined to make a food that is on the FTL, a traceability lot code must be linked to KDEs related to location and date of creation, quantity and various other records related to the creation of the food. For shipping, persons who ship food from the FTL need to maintain records including the traceability lot code and KDEs related to shipping the food. There are additional shipping KDE’s if the shipper is a farm.
Record Keeping
In order to maintain appropriate recordkeeping, persons who engage in production of a food on the FTL must create and maintain traceability program records. These records should assist regulators in comprehending a producers’ traceability program. The records include: a description of relevant reference records; a list of foods on the FTL that are shipped; a description of how traceability lot codes are assigned; and other information needed to understand data provided within the required records.
Exemptions
The proposed rule includes several exemptions, excluding produce that is rarely consumed raw; farms that sell directly to consumers; certain food produced and packaged on farm; and several others. The FDA specifically calls out the exemption for small retail food establishments multiple times in their guidance documents. For small retail food establishments, the FDA has two approaches to this exemption: a full exemption and a partial exemption. Both exemptions apply to establishments with fewer than ten full-time equivalent employees. The full exemption exempts an establishment from all requirements of the proposed rule. The partial exemption exempts small retail food establishments with fewer than ten full time equivalent employees from the requirement to provide FDA an electronic sortable spreadsheet with traceability information. However, this partial exemption does not apply to the other parts of the rule. There are several other situations in which a type partial exemption is granted, such as a partial exemption for food from fishing vessels. The proposed rule also provides a process for entities to request modified requirements and additional exemptions. Further, the proposed rule allows the FDA to issue waivers in certain circumstances where economic hardship exists.
Conclusion
The proposed traceability rule aims to reduce the time it takes to respond to a foodborne illness outbreak and identify the exact point of creation. By enhancing traceability for these high-risk foods, the impact of foodborne illness outbreaks can be lessened and breadth of recalls can be reduced. Through collaboration with industry stakeholders in the pilot programs, the FDA believes they are created a rule that will be applicable to different industries and parts of the supply chain. The FDA has also left some flexibility in the rule with the various exemptions, requests for special exemptions and the ability to issue waivers. Further, FDA has emphasized that this rule can be adopted using various technologies and does not require the use of blockchain (although blockchain would be permitted). This proposed rule is available for comment until February 22, 2021.
For more information on the FSMA Pilot Projects, click here.
For more information on the leafy greens pilot project, click here.
For more information on the Food Traceability List, click here.
For more information on the proposed rule, click here.
To view materials from the NALC’s webinar on blockchain, click here.